GMP/EPCM service
-
Guangzhou Westpoint Pharmatech. Co., Ltd.
Tel:0769-2223 5501
Address: 4th Floor, 8th Building, Industrial Center, No. 19 Alishan Road, Songshan Lake High-tech Industrial Development Zone, Dongguan City, Guangdong Province
Dongguan Westpoint Pharmatech. Co., Ltd.
Tel:0769-2223 5501
Address: 4th Floor, 8th Building, Industrial Center, No. 19 Alishan Road, Songshan Lake High-tech Industrial Development Zone, Dongguan City, Guangdong Province
Jiangsu Westpoint Pharmaceutical Excipients Co., Ltd.
Tel:0523-80103166
Address: West side of 1st to 4th floor of G56 Standard Factory Building of China Pharmaceutical City Phase IV, Hailing District, Taizhou City, Jiangsu Province
Your location:Home > Company's Profile
Technology Transfer Inc. (USA), the predecessor of Guangzhou WestPoint Pharmatech. Co., Ltd., has been the first European and American consulting company in China since 1983. As a comprehensive pharmaceutical technology and regulatory compliance consulting company, Technology Transfer Inc. had 30 years of consulting service in US, providing services on manufacture facility construction and technical/regulation for pharmaceutical enterprises around the world. The completed projects include the design, construction, validation and GMP certification for more than 40 major projects from the United States to Canada in North America, Russia, Poland, Britain, Spain in Europe,Egypt, Jordan in the Middle East, India, Pakistan, Indonesia and other countries and regions in Southeast Asia.
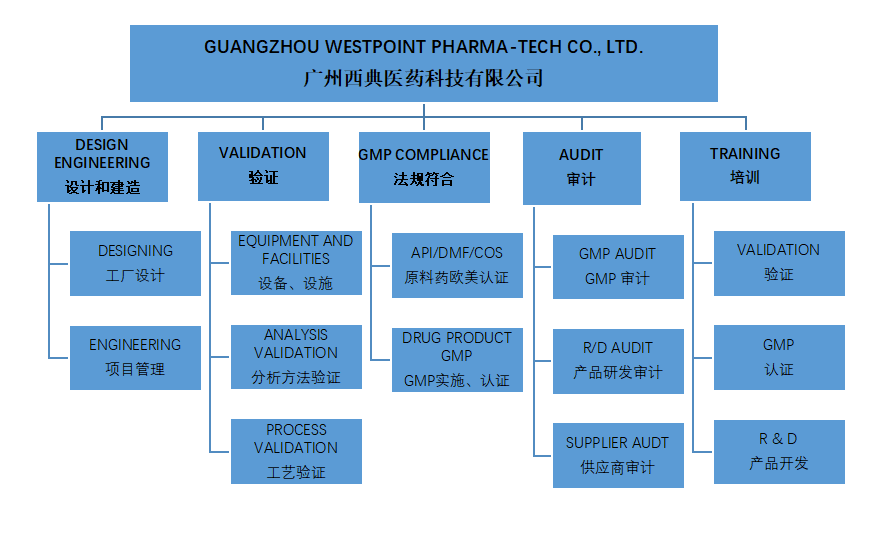
Guangzhou WestPoint Pharmatech. Co., Ltd. focuses on the implementing global standard GMP and the general contract for project management of pharmaceutical engineering construction (including: project plan, design, construction, validation, product technology transfer, GMP certification process management). The initial service clients are mainly European and American joint ventures in China. According to the construction standards of European and American pharmaceutical enterprises, our company proposed and implemented facility design/construction plan, project management, validation and GMP system compliance. Our mid-term services were mainly for domestic enterprises which were ready to enter the global market following EU and US drug regulation and GMP requirements. In order to meet the needs of domestic drug products entering into European and American markets, we need to carry out facility planning and to establish GMP system. In the recent development trend of Chinese pharmaceutical industry's overall global integration, our company will help domestic enterprises to improve their technical and management skills in compliance with GMP requirements of Europe, America and China.



 0769-22235501
0769-22235501
