GMP/EPCM service
-
Guangzhou Westpoint Pharmatech. Co., Ltd.
Tel:0769-2223 5501
Address: 4th Floor, 8th Building, Industrial Center, No. 19 Alishan Road, Songshan Lake High-tech Industrial Development Zone, Dongguan City, Guangdong Province
Dongguan Westpoint Pharmatech. Co., Ltd.
Tel:0769-2223 5501
Address: 4th Floor, 8th Building, Industrial Center, No. 19 Alishan Road, Songshan Lake High-tech Industrial Development Zone, Dongguan City, Guangdong Province
Jiangsu Westpoint Pharmaceutical Excipients Co., Ltd.
Tel:0523-80103166
Address: West side of 1st to 4th floor of G56 Standard Factory Building of China Pharmaceutical City Phase IV, Hailing District, Taizhou City, Jiangsu Province
Your location:Home > Verification
Verification
The validation system is a high-tech work. Following the relevant GMP regulation, the technical requirements of validation have been constantly raised. It is the most complicated work with the heaviest workload and the highest technical standards for a new pharmaceutical facility.
The filing system of the validation includes the hardware system validation around the facility construction, the product manufacture process validation and the test methods validation about the drug product quality. The filing structure is as follows:
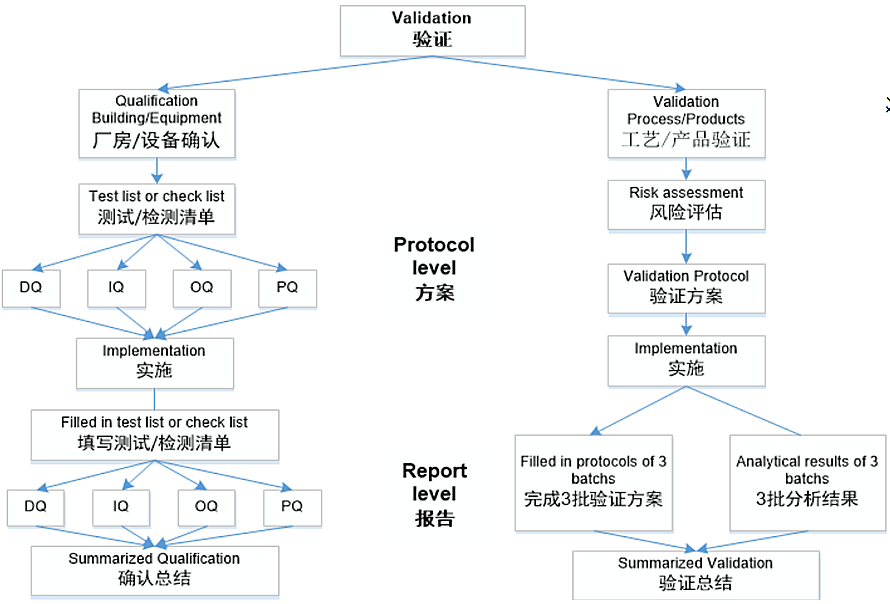
The validation system is constantly expanding and improving. According to the requirements of current GMP regulation, the validation system is redefined and standardized and the interactions of individual sector during validation are as below:
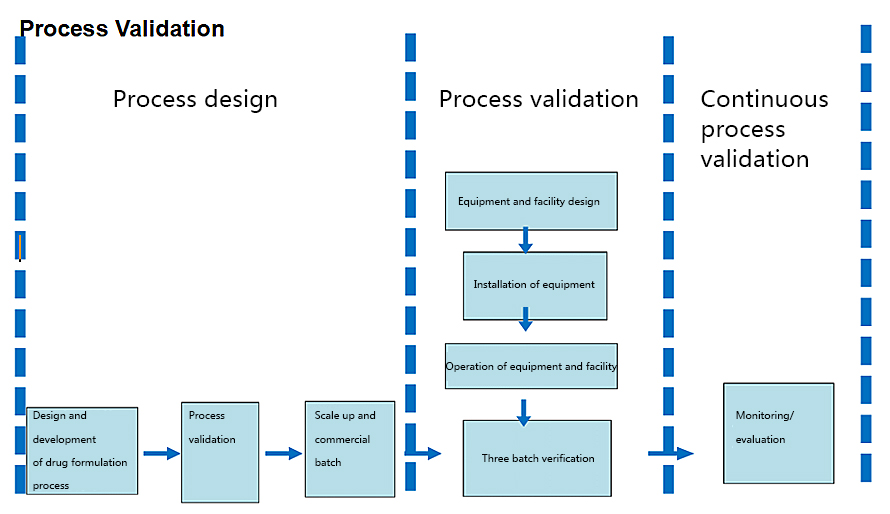
Currently there are always serious deficiencies of process validation in domestic pharmaceutical enterprises. The establishment of a validation system is to ensure the satisfied production technology and quality of drug products. All these issues are so important to the management of pharmaceutical enterprises and should be given full attention.
Validation relies on a fully understanding of the technology, quality control of product, regulatory requirements, scientific and rational test design, suitable analytical methods and data summarization. We can design validation systems for different enterprises, different products, different regulatory requirements and different management target. We can also assist/or implement them.
Validation
Quality of validation and confirmation
>Documents of GMP validation system in EU, US and China;
>Standardized implementation protocol of validation system;
>Validation techniques and methods that meet the GMP requirements in EU/US;
>Validation is a very technical work. The method and technical approach will determine the quality of validation.
Chinese traditional level > Global GMP certification level >
EU/US level
Implementation methods for validation and confirmation
>Objectives: ensure the company is at a high GMP level;
>Risk assessment and control;
>Determination of the objectives and contents of validation;
>Experimental design for validation and confirmation;
>Preparation and execution of validation protocol;
>Validation of data process and final report.
Confirm the quality of facility and engineering
>Documents for project quality confirmation;
>Facility confirmation;



 0769-22235501
0769-22235501
